Compound Overview
Class of Compound:
Peptide
Mechanism of Action:
Tirzepatide is a dual GIP and GLP-1 receptor agonist, mimicking the effects of endogenous GIP while inducing cAMP synthesis at the GLP-1 receptor. This unique mechanism induces a synergistic effect with enhanced insulin response and glucagonostatic activity, versus GIP or GLP-1 therapy alone.
Notable Studies:
Also Known As:
LY3298176, GIP/GLP-1 RA
Research Applications:
- Weight Loss
- Glycemic Control
- Lipid Profile Improvement
Risks:
- Gastrointestinal Side Effects
- Hypersensitivity Reactions
- Thyroid C-Cell Tumors in Rodent Studies
Chemical Structure
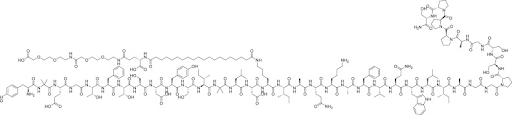
What is Tirzepatide?
Tirzepatide, or LY3298176, is a unimolecular dual glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor agonist that is under study for its glycemic control and weight loss benefits [1, 2, 3].
Tirzepatide is based on the human GIP hormone and has a length of 39 amino acids. It includes a C20 fatty di-acid moiety that provides extended duration and allows for once-weekly dosing in humans [1, 2, 3].
Following extensive clinical testing, including against comparable treatments, tirzepatide was approved in May 2022 by the United State Food and Drug Administration (USFDA) as a type 2 diabetes (T2D) treatment. It became the first dual GLP-1 and GIP receptor agonist indicated for T2D [4, 5].
Tirzepatide is now in clinical trials to establish its safety and efficacy as a weight loss treatment in adults with a body mass index (BMI) of 27 or greater, with the initial results suggesting that it provides significant and sustained weight management benefits [6, 7].
While it is now an approved prescription medication for T2D patients, tirzepatide is also available as a reference material to credentialed professionals wishing to conduct research on this novel GLP-1/GLP agonist.
What Does Tirzepatide Do?
Tirzepatide is a potent instrument for controlling both weight and blood sugar, thanks to its dual GLP-1/GLP action. Researchers have found that its dual receptor agonism produces a synergistic effect for significantly enhanced insulin response and glucagonostatic activity, versus administering either GIP or GLP-1 monotherapy [3, 5].
GIP and GLP-1 are the two primary incretin hormones that the intestine secretes upon ingestion of glucose or nutrients, with the effect of stimulating insulin secretion from pancreatic beta cells [8]. Tirzepatide has similar affinity at the GIP receptor as native GIP, but has five times lower GLP-1 receptor affinity compared to native GLP-1. In the latter, tirzepatide has a preference for cAMP signaling over beta-arrestin recruitment [9].
This biased agonism of tirzapetide and unique GLP-1 signaling are believed to underlie its efficacy in enhancing insulin secretion [5]. In fact, clinical trials have demonstrated tirzepatide’s superior efficacy and comparable safety as a glucose lowering agent when compared to established T2D treatments [10, 11, 12, 13, 14].
Researchers should also note tirzepatide’s ability to significantly increase adiponectin, an adipokine linked to lipid and glucose metabolism regulation [1, 15]. Increases of serum adiponectin have been associated with weight loss, exercise, and improved nutrition. Researchers believe that this particular mechanism of tirzepatide has cardioprotective implications [16].
Tirzepatide Benefits | Clinical Trials
While tirzepatide has already been approved as a safe and effective treatment of type 2 diabetes, researchers are now intrigued by its potential weight loss and cardioprotective benefits.
Clinical trials to investigate these uses are ongoing until at least 2024. Accordingly, we will summarize only the results published as of the date of writing.
Tirzepatide and Weight Loss: The ability of tirzepatide to induce weight loss is at least partially explained by its mechanism of activating GIP receptors in fat cells, resulting in decreased adipose inflammation and increased adiponectin, both associated with reduced fat cell differentiation and increased energy expenditure [1, 17].
In view of the global obesity crisis and the fairly limited treatment options on the market, Eli Lilly and Company, as tirzepatide patent holder, kicked off a clinical development program to test tirzepatide as a weight control agent in late 2022. Called SURMOUNT, the program is set to include four global phase 3 trials to evaluate tirzepatide’s safety and efficacy as an adjunct weight loss treatment in obese and overweight adults, defined as those with a BMI of ≥ 27 [6].
The first trial numbered over 2,500 participants and confirmed the peptide’s efficacy in inducing weight loss in obese and overweight patients. Study authors observed average weight reductions of 16% for patients on tirzepatide 5mg/weekly, 21.4% for tirzepatide 10mg/weekly, and 22.5% for tirzepatide 15mg/weekly, over the course of 72 weeks. The remaining trials are set to conclude in 2023 [6, 7].
Tirzepatide as a T2D Treatment: BAs discussed, tirzepatide’s dual GLP-1/GLP action sets it apart as a T2D treatment [3, 5].
Researchers believe that its unique mechanism of action, summarized as mimicking native GIP at the GIP receptor while exhibiting bias at the GLP-1 receptor for cAMP generation over beta-arrestin recruitment, underlies its efficacy as an anti-diabetes drug [9].
Research has shown that tirzepatide is superior to other diabetes therapies like semaglutide and dulaglutide in terms of glycemic control and weight loss. For example, researchers discovered that tirzepatide was more effective than semaglutide at lowering hemoglobin A1c and causing weight loss in T2D patients [11, 18].
On the basis of its comparative effectiveness, the USFDA has approved tirzepatide as an adjunct treatment for improving blood sugar control in adults with T2D, as an addition to doctor-supervised diet and exercise [4].
Tirzepatide and Cardioprotective Benefits: Research surrounding GLP-1 shows that the incretin hormone is key to directly regulating risk factors like hypertension and obesity, while indirectly regulating risk factors like inflammation and endothelial cell dysfunction. It is thus believed that tirzepatide’s selective targeting of the GLP-1 receptor may slow the development and progression of cardiovascular complications, particularly in diabetic patients [19].
In a 26-week study on T2D patients, once-weekly tirzepatide injections improved lipoprotein biomarkers associated with insulin resistance and cardiovascular risk, while reducing triglycerides, suggesting a net lowering of the patients’ risk of heart disease [20].
A pending cardiovascular outcomes study should provide a more accurate picture of the cardioprotective benefits of tirzepatide, pitting it against the GLP-1 receptor agonist dulaglutide [21].
Buy Tirzepatide from our top-rated vendor...

Tirzepatide Side Effects
Based on the data available to date, tirzepatide is well-tolerated and not linked to any serious adverse effects in obese or overweight adults with or without type 2 diabetes [7, 10, 11, 12, 13, 14].
Researcheres should note that tirzepatide administration may produce minor side effects, usually GI tract-related. These will generally cease with discontinuation of therapy or after lowering the total dosage.
Here is a non-exhaustive list of tirzepatide side effects observed to date [7, 10, 11, 12, 13, 14]:
- Nausea
- Vomiting
- Diarrhea
- Reduced appetite
- Constipation
- Indigestion
- Dyspepsia
- Abdominal pain
- Hypersensitivity reactions
Researchers should note that tirzepartide has a gastric emptying effect, and are therefore advised to refrain administering the peptide to subjects with severe gastrointestinal disease [22].
Is Tirzepatide Safe?
Based on the SURPASS clinical development program, the United States Drug Administration has approved tirzepatide as a treatment of type 2 diabetes, considering it safe for use in adults with this condition [10, 11, 12, 13, 14].
In the phase 3 SURPASS-1 trial, researchers found tirzepatide to have a safety profile comparable to that of other GLP-1 receptor agonists [10]. In the phase 3 SURPASS-4 trial, the study authors ruled out excess cardiovascular risk as a safety concern of tirzepatide in T2D patients [11].
Safety studies for other uses of tirzepatide are still ongoing, and research into tirzerpatide’s use as a weight loss treatment (the SURMOUNT program) already indicates a favorable safety profile [6, 7].
Where sold as a reference material or research chemical, tirzepatide must be handled only by qualified researchers and authorized laboratory personnel, bearing in mind the limitations on safety data to date.
Researchers may consult the Mounjaro (tirzepatide) package insert for details on the safe and effective administration of tirzepatide. We summarize relevant points on the peptide’s safe administration [23]:
- Tirzepatide is administered by subcutaneous injection, into the fatty tissue beneath the skin (usually in the abdomen). It is best practice to rotate the injection site with each injection.
- Tirzepatide should not be administered to subjects who have a history of medullary thyroid carcinoma, as it has been found to cause thyroid C-cell tumors in rats. Tirzepatide is also contraindicated in subjects with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- Subjects who are receiving insulin or an insulin secretagogue together with tirzepatide should be monitored for hypoglycemia.
- The peptide may delay absorption of certain oral medications. Subjects who are being administered drugs with a narrow therapeutic index should also be monitored.
Tirzepatide Dosage Calculator
As a reference material, tirzepatide may be studied for a range of clinical applications and at varying doses depending on the research performed.
Researchers should note that the current recommendations for tirzepatide reflect the populations studied in clinical trials — patients suffering from type 2 diabetes and/or obesity [7, 10, 11, 12, 13, 14].
In determining the correct tirzepatide dosage for their research, investigators may also refer to the label recommendations published in the Mounjaro (tirzepatide) package insert [23].
Based on the available information to date, here is a reference tirzepatide dosing protocol to induce weight loss in obese or overweight subjects:
- Starting Dose (Weeks 1-4): 2.5mg/week for the first four weeks of the study period.
- Dose Increase (Weeks 5-24): Increase total weekly dose to 5mg. Evaluate response and further increase the total weekly dose in 2.5/mg increments, as needed.
- Frequency: Once per week, subcutaneous.
- Duration: 12-24 weeks.
- Notes: The maximum established weekly dose is 15mg, per the Mounjaro dosing guidelines and clinical trial data.
Where to Buy Tirzepatide Online? | 2024 Edition
Tirzepatide is available for sale online to peptide researchers and laboratory professionals.
Given the sheer number of vendors now listing tirzepatide and other research peptides for sale, researchers are well-advised to carefully go over their options before making a purchase.
At Peptides.org, we consistently turn to one tirzepatide vendor that meets our standards and more:
Golden Peptides
Golden Peptides is a vendor of top-quality research chemicals, including a range of weight loss peptides, growth hormone secretagogues, and longevity compounds.
They offer tirzepatide exclusively to their members. Signup is easy and access is nearly instant.
Here is what researchers can expect when buying tirzepatide from this top source:
- USA-Made Tirzepatide: The vendor works only with accredited laboratories in the USA to ensure product quality, providing assurance that their peptides are produced and packed in line with industry best practices.
- Lab-Tested Peptides: Golden Peptides is committed to providing 99%+ purity peptides, as documented by HPLC-MS testing results provided by a reputable third-party laboratory.
- Customer Care: The vendor stands by its commitment to customer satisfaction, and is quick to resolve any errors or mistakes in the shipping process.
- Free U.S. Shipping ($200+): Golden Peptides waives domestic shipping on purchases of $200+, and most U.S. orders are shipped on the same day of payment receipt.
Known for their top-quality peptides, fair prices, and customer-first approach, Golden Peptides is the one-stop source for tirzepatide and related peptides.
Click the link below to visit Golden Peptides for instant access to 99% pure tirzepatide and other weight loss peptides for research:
Buy research peptides from GOLDEN Peptides today...

Bacteriostatic Water and Tirzepatide
To incorporate tirzepatide, as well as all other research peptides, into an experiment, the lab needs to be set up with the right supplies.
For both efficacy and safety when carrying out peptide reconstitution and storage, researchers need access to items like insulin syringes, sterile vials, and bacteriostatic water, among others.
Rather than spending time and energy trying to source all these items from various retailers, the experts at Peptides.org recommend that researchers pick up some supplies from our top-recommended vendor.
FAQ
Tirzepatide. Just. Works.
Tirzepatide is a first-in-class T2D medication that activates both the GIP and GLP-1 receptors to help patients better manage blood sugar, while triggering weight loss and improving lipid markers.
The novel drug has also yielded positive results in clinical trials on overweight and obese subjects, and further data on tirzepatide’s role in weight loss is eagerly awaited.
Tirzepatide’s favorable safety profile in T2D and obese patient groups makes it a promising candidate for further research in related clinical applications.
Qualified researchers who wish to buy tirzepatide online should look to none other than our go-to source of research-grade peptides.
References
- Ali, Rouchan & Virendra, Sharma & Chawla, Pooja. (2022). Bumps and humps in the success of Tirzepatide as the first GLP1 and GIP receptor agonist. Health Sciences Review. 4. 100032. 10.1016/j.hsr.2022.100032.
- Thomas MK, Nikooienejad A, Bray R, et al. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. J Clin Endocrinol Metab. 2021;106(2):388-396. doi:10.1210/clinem/dgaa863
- Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB, Cui X, Briere DA, Cabrera O, Roell WC, Kuchibhotla U, Moyers JS, Benson CT, Gimeno RE, D'Alessio DA, Haupt A. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab. 2018 Dec;18:3-14. doi: 10.1016/j.molmet.2018.09.009. Epub 2018 Oct 3. PMID: 30473097; PMCID: PMC6308032.
- Office of the Commissioner. (2022, May 13). FDA Approves Novel, Dual-Targeted Treatment for Type 2 Diabetes. U.S. Food And Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-novel-dual-targeted-treatment-type-2-diabetes
- Min T, Bain SC. The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials. Diabetes Ther. 2021;12(1):143-157. doi:10.1007/s13300-020-00981-0
- le Roux CW, Zhang S, Aronne LJ, Kushner RF, Chao AM, Machineni S, Dunn J, Chigutsa FB, Ahmad NN, Bunck MC. Tirzepatide for the treatment of obesity: Rationale and design of the SURMOUNT clinical development program. Obesity (Silver Spring). 2023 Jan;31(1):96-110. doi: 10.1002/oby.23612. Epub 2022 Dec 7. PMID: 36478180.
- Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, Stefanski A; SURMOUNT-1 Investigators. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jul 21;387(3):205-216. doi: 10.1056/NEJMoa2206038. Epub 2022 Jun 4. PMID: 35658024.
- Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig. 2010 Apr 22;1(1-2):8-23. doi: 10.1111/j.2040-1124.2010.00022.x. PMID: 24843404; PMCID: PMC4020673.
- Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, Capozzi ME, van der Velden WJ, Stutsman C, Cardona GR, Urva S, Emmerson PJ, Holst JJ, D'Alessio DA, Coghlan MP, Rosenkilde MM, Campbell JE, Sloop KW. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. 2020 Sep 3;5(17):e140532. doi: 10.1172/jci.insight.140532. PMID: 32730231; PMCID: PMC7526454.
- Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, Mao H, Cui X, Karanikas CA, Thieu VT. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021 Jul 10;398(10295):143-155. doi: 10.1016/S0140-6736(21)01324-6. Epub 2021 Jun 27. Erratum in: Lancet. 2021 Jul 17;398(10296):212. PMID: 34186022.
- Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021 Aug 5;385(6):503-515. doi: 10.1056/NEJMoa2107519. Epub 2021 Jun 25. PMID: 34170647.
- Ludvik, B., Giorgino, F., Jódar, E., Frias, J. P., Landó, L. F., Brown, K., Bray, R., & Rodríguez, Á. (2021, August 6). Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (surpass-3): A randomised, open-label, parallel-group, phase 3 trial. The Lancet. Retrieved August 9, 2022, from https://www.sciencedirect.com/science/article/abs/pii/S0140673621014434
- Prato, S. D., Kahn, S. E., Pavo, I., Weerakkody, G. J., Yang, Z., Doupis, J., Aizenberg, D., Wynne, A. G., Riesmeyer, J. S., Heine, R. J., & Wiese, R. J. (2021, October 18). Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (surpass-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. The Lancet. Retrieved August 9, 2022, from https://www.sciencedirect.com/science/article/abs/pii/S0140673621021887
- Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, Rodríguez Á. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA. 2022 Feb 8;327(6):534-545. doi: 10.1001/jama.2022.0078. PMID: 35133415; PMCID: PMC8826179.
- Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, Milicevic Z, Haupt A, Robins DA. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. J Clin Endocrinol Metab. 2021 Jan 23;106(2):388-396. doi: 10.1210/clinem/dgaa863. PMID: 33236115; PMCID: PMC7823251.
- Yanai H, Yoshida H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. International Journal of Molecular Sciences. 2019; 20(5):1190. https://doi.org/10.3390/ijms20051190
- Zhang, Qian et al. “The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling.” Cell metabolism vol. 33,4 (2021): 833-844.e5. doi:10.1016/j.cmet.2021.01.015
- Vadher, Karan et al. “Efficacy of tirzepatide 5, 10 and 15 mg versus semaglutide 2 mg in patients with type 2 diabetes: An adjusted indirect treatment comparison.” Diabetes, obesity & metabolism, 10.1111/dom.14775. 19 May. 2022, doi:10.1111/dom.14775
- Tate M, Chong A, Robinson E, Green BD, Grieve DJ. Selective targeting of glucagon-like peptide-1 signalling as a novel therapeutic approach for cardiovascular disease in diabetes. Br J Pharmacol. 2015;172(3):721-736. doi:10.1111/bph.12943
- Wilson, JM, Nikooienejad, A, Robins, DA, et al. The dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist, tirzepatide, improves lipoprotein biomarkers associated with insulin resistance and cardiovascular risk in patients with type 2 diabetes. Diabetes Obes Metab. 2020; 22: 2451– 2459.
- National Library of Medicine (U.S.). (2020, May 29 - ). A Study of Tirzepatide (LY3298176) Compared With Dulaglutide on Major Cardiovascular Events in Participants With Type 2 Diabetes (SURPASS-CVOT). Identifier NCT04255433. https://www.clinicaltrials.gov/ct2/show/NCT04255433
- Frias JP, Nauck MA, Van J, Benson C, Bray R, Cui X, Milicevic Z, Urva S, Haupt A, Robins DA. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes Obes Metab. 2020 Jun;22(6):938-946. doi: 10.1111/dom.13979. Epub 2020 Feb 11. PMID: 31984598; PMCID: PMC7318331.
- Highlights of prescribing information ... - eli lilly and company. (n.d.). Retrieved January 10, 2023, from https://pi.lilly.com/us/mounjaro-uspi.pdf?s=pi
