Compound Overview
Class of Compound:
Peptide
Mechanism of Action:
N-Acetyl Selank Amidate passes through the blood-brain barrier and acts on the central nervous system by interacting with BDNF levels and opioid, serotonergic, and GABA signaling. This may result in anxiolytic, stress-reducing, and nootropic effects.
Notable Studies:
- Efficacy and possible mechanisms of action of a new peptide anxiolytic selank in the therapy of generalized anxiety disorders and neurasthenia
- Optimization of the treatment of anxiety disorders with selank
- A comparison of the anxiolytic effect and tolerability of selank and phenazepam in the treatment of anxiety disorders
Also Known As:
Ac-Thr-Lys-Pro-Arg-Pro-Gly-Pro-NH2
Research Applications:
- Anxiety Reduction
- Cognitive Enhancement
- Mood Regulation
Risks:
- Lack of Research Outside of Russia
- Little Safety Data
- Lack of Regulatory Oversight
Chemical Structure
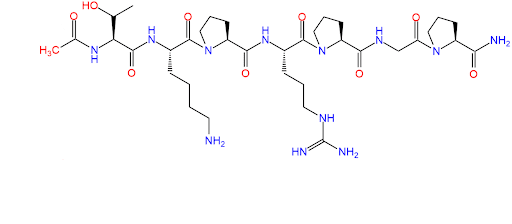
What is N-Acetyl Selank Amidate?
N-Acetyl Selank Amidate is a modified version of the heptapeptide Selank, which was first developed in the 1990s at the Institute of Molecular Genetics of the Russian Academy of Sciences [1].
Like unmodified Selank, N-Acetyl Selank Amidate is also a heptapeptide and a synthetic analog of the endogenous human tetrapeptide tuftsin (threonine - lysine - proline - arginine).
N-Acetyl Selank Amidate has the following modifications in relation to Selank:
- The peptide’s C-terminus is modified by the addition of a Pro-Gly-Pro fragment to enhance its ability to pass the blood-brain barrier (BBB) [2]. This allows the peptide to act on the central nervous system (CNS) when applied via injection or intranasally [3].
- N-Acetyl Selank Amidate has an additional acetyl group attached to the N-terminus and an amide group attached to the C-terminus. These modifications are believed to protect the N- and C-terminal groups from breakdown and increase the stability of peptides such as Selank [4, 5].
There is a lack of research regarding the potential effects of N-Acetyl Selank Amidate. Still, it is believed to act similarly to Selank.
Selank was approved for human use by the Russian Federation Ministry of Health in 2009 and is currently available by prescription only in Russia and Ukraine [1]. Based on its effects on the CNS, the peptide is approved for use in generalized anxiety disorder (GAD) in those countries.
Selank, and potentially N-Acetyl Selank Amidate, may also exert nootropic effects thanks to its ability to reduce stress perception. It has also been noted to improve memory retention in murine models [6, 7].
As an analog of the immunomodulatory peptide tuftsin, Selank has been studied for its potential immunomodulatory properties in addition to its anxiolytic and nootropic effects [8].

What Does N-Acetyl Selank Amidate Do?
As of writing, there is little data on the mechanisms of N-Acetyl Selank Amidate specifically.
But since it is a version of Selank with modifications aimed at improving its stability rather than changing its effects, it is reasonable to infer that N-Acetyl Selank Amidate may have similar mechanisms to its parent molecule.
According to the available data, Selank exerts its effects by passing through the BBB and interacting with certain neurotrophic factors and neurotransmitters in the CNS, including:
- Gamma amino butyric acid (GABA): Studies reveal that Selank may act as a positive allosteric modulator of GABA signaling. GABA is an inhibitory neurotransmitter in the brain that reduces neuronal excitability, promotes relaxation, and alleviates anxiety symptoms [2].
- Serotonin: According to research in laboratory animal models, Selank enhances serotonin metabolism in the brain stem 30 minutes after administration. Serotonin signaling in the brain helps to regulate mood and anxiety by enhancing feelings of well-being and relaxation [9].
- Enkephalins: Studies report that Selank may block the activity of enkephalin-degrading enzymes and thus upregulate enkephalin levels [10]. Enkephalins are the physiological ligands of opioid receptors, and apart from their role in pain perception, they may benefit mood and reduce stress when upregulated [11]. Selank’s ability to upregulate enkephalins has also been demonstrated in clinical studies [12].
- Brain-derived neurotrophic factor (BDNF): Intranasal administration of Selank has also been shown to upregulate BDNF expression in the hippocampal area in murine models [13]. BDNF is a neurotrophic factor that promotes the growth and survival of neurons, enhancing synaptic plasticity and improving overall brain function. This can contribute to improvements in cognition, mood, and anxiety symptoms.
Additional studies suggest that Selank may affect dopamine and norepinephrine signaling, but research is inconclusive. Selank’s interactions with these neurotransmitters may at least partially explain its anxiolytic effects [14].
Research Applications and Benefits of N-Acetyl Selank Amidate
While there is currently a lack of research into N-Acetyl Selank Amidate specifically, it is expected to have similar effects and potentially enhanced benefits compared to Selank peptide.
Accordingly, we will now outline the key benefits of Selank as observed in research studies.
N-Acetyl Selank Amidate for Stress and Anxiety Reduction
Selank has been clinically studied for its anxiolytic and anti-stress potential.
Most notably, one noninferiority trial in 62 patients with generalized anxiety disorder (GAD) sought to compare the anxiolytic action of intranasal Selank to that of medazepam, an antidepressant and anxiolytic drug. Here are the most notable findings reported by the study’s authors [12]:
- Selank appears to have similar efficacy in reducing symptoms of GAD as the established anxiolytic medazepam.
- GAD appeared to be associated with decreased tau (1/2) leu-enkephalin levels. The application of Selank offset the decrease.
- In addition to Selank’s potent anxiolytic effects, the peptide was also observed to have antiasthenic and psychostimulatory properties.
N-Acetyl Selank Amidate for Cognitive Enhancement
Clinical studies on Selank suggest that the peptide may have nootropic effects, namely in research subjects with anxiety-related disorders.
Some researchers have even suggested that the peptide may potentiate the benefits of common anxiolytic medications while reducing the risk of side effects. Here are the most notable study findings on the topic:
- A study in 70 anxiety disorder patients applied either oral phenazepam alone or in combination with intranasal Selank. The study authors reported that Selank significantly enhanced the anxiolytic medication’s effectiveness. The combination therapy also mitigated common side effects often associated with benzodiazepines, including memory loss, attention deficits, asthenia, and drowsiness [15].
- Another study in 60 anxiety disorder patients reported that Selank therapy led to mild nootropic effects and pronounced anxiolytic potential [6].
- Murine studies have also suggested that Selank may improve serotonin metabolism in the CNS while enhancing memory storage processes [7].
N-Acetyl Selank Amidate and Immunomodulation
Selank appears to also have immunomodulatory effects, particularly under stress conditions and in mood disorders such as depression and generalized anxiety disorder.
Here are two of the most noted studies on the topic:
- One study in stressed laboratory animals reported that Selank effectively reduced concentrations of pro-inflammatory cytokines like IL-1β, IL-6, and TGF-β1 while restoring levels of anti-inflammatory cytokine IL-4. This suggests that Selank has stress-protective activity and could modulate stress-related immune responses [16].
- A clinical study reported that 14 days of Selank administration suppressed the gene expression of IL-6 in the peripheral blood of patients with depression. Moreover, the peptide altered the Th1/Th2 cytokine balance in GAD patients to a more favorable ratio, indicating its potential as an immunomodulator in anxiety disorders [17].
Buy N-Acetyl Selank from our top-rated vendor...

Side Effects of N-Acetyl Selank Amidate
Studies on the safety and side effects of N-Acetyl Selank Amidate are lacking. Yet, researchers have explored the side effects of intranasally administered Selank, which may be used as a reference.
The data on Selank indicate a high level of tolerability and an absence of toxicity for durations of up to one month of administration.
Additionally, some research suggests that Selank could mitigate the adverse effects of other anxiety-reducing substances like benzodiazepines [15].
However, minor side effects may arise from the nasal use of peptides, such as N-Acetyl Selank Amidate. The most prevalent ones are listed below:
- Nasal dryness
- Throat irritation
- Hypersensitivity
- Bacterial contamination
If administered via subcutaneous injections, peptides like N-Acetyl Selank Amidate may also lead to the following reactions at the site of injection:
- Pain
- Induration
- Bleeding
- Redness
- Swelling
While some research has pointed to a link between elevated BDNP and a potentially increased hair loss risk in patients predisposed to baldness, it is currently unclear whether Selank, which boosts BDNF, carries this risk [18].
Is N-Acetyl Selank Amidate Safe?
As of writing, safety data on N-Acetyl Selank Amidate are lacking. Based on the data gathered by Selank researchers, the peptide appears to be safe for short-term use. Selank is notably approved for intranasal use in the Russian Federation.
Some authors posit that long-term Selank use may cause desensitization of the GABAergic system, resulting in increased anxiety, depression, insomnia, and related issues. Yet, current data does not definitively support this concern [19].
Nevertheless, researchers should lean on the side of safety and discontinue applying the peptide following 2-3 weeks of application.
Keep reading to learn more about the latest guidelines on N-Acetyl Selank Amidate dosing.

N-Acetyl Selank Amidate Dosing | Research Only
Due to the lack of research, there are no official N-Acetyl Selank Amidate dosing guidelines.
Yet, intranasal Selank has been studied extensively and is prescribed in Russia and Ukraine for the management of GAD.
Here are some key points related to Selank dosage, which may be used as a reference for dosing the modified N-Acetyl Selank Amidate version:
- Dosage according to the package insert: 0.15% Selank nasal drops may be applied daily for up to 14 days. The dosage is two drops (75mcg per drop) given three times daily. After completing a 14-day cycle, a break of one to three weeks is recommended before any repeat application [20].
- Dosage according to clinical trials: In clinical studies, Selank has been administered intranasally in doses of up to 2700mcg (equivalent to 36 drops) divided into three daily applications for up to 21 days, followed by a washout period [15].
To mitigate the risk of receptor desensitization, experts suggest a one to three-week washout period following a 14-day course of Selank [20]. Thus, researchers investigating N-Acetyl Selank Amidate are also recommended to adhere to this guideline.
Here is a sample dosing protocol for N-Acetyl Selank based on the available data on Selank, and considering a N-Acetyl Selank Amidate nasal spray for research that delivers 300mcg per pump:
- Daily Dosage: Begin with one pump applied 2-3 times/daily for a total of 600-900mcg/daily intranasal of N-Acetyl Selank Amidate. Increase the dose as needed.
- Frequency: Administer two or three times a day at regular intervals.
- Duration of Study: Up to 14 days, followed by a minimum of one week off. Another two-week course may be initiated as needed.
- Monitoring and Safety: Closely monitor subjects for any adverse reactions. If any negative effects are observed, cease N-Acetyl Selank Amidate administration immediately.
Where to Buy N-Acetyl Selank Amidate Online? | 2024 Edition
Peptide researchers can legally source N-Acetyl Selank Amidate online as a reference material.
We strongly urge researchers to make their purchases exclusively from verified suppliers. Here is our top recommendation for N-Acetyl Selank Amidate nasal spray.
Limitless Life
Our team endorses Limitless Life as a trustworthy provider of peptide nasal sprays, including N-Acetyl Selank Amidate, for these reasons:
- Independent Lab Analysis: Limitless Life commits to providing only research-grade peptides, including N-Acetyl Selank Amidate. The vendor has each peptide batch rigorously examined by an external lab to meet high purity levels.
- Prompt Delivery & Free Domestic Shipping: Limitless Life offers free shipping on U.S. orders of $350+ and ensures rapid order processing, often dispatching purchases on the day they are made.
- Exceptional Client Support: Researchers can reach out to Limitless Life’s trained professional and responsive customer service team by phone or email.
Buy N-Acetyl Selank Amidate nasal spray from Limitless Life to ensure validity and success in research studies.
Buy N-Acetyl Selank from our top-rated vendor...

N-Acetyl Selank Amidate Nasal Spray | A-Z Guide
N-Acetyl Selank Amidate nasal spray is typically formulated using a reconstituted solution in a sterile spray bottle that is ready to use.
As such, it must be stored at 36 to 46 degrees F (2 to 8 degrees C) at all times.
Here is a simple step-by-step guide to administering N-Acetyl Selank Amidate nasal spray in research settings:
- Disinfect hands, and wipe the nozzle of the N-Acetyl Selank Amidate spray bottle with an alcohol swab.
- Ensure the solution is well mixed by gently rolling it between the hands without shaking.
- Clear the subject’s nasal cavity and identify the more open nostril.
- With the non-dominant hand, gently close the target nostril.
- Hold the spray bottle in the dominant hand, slightly tilting the subject’s head. Insert the nozzle deep into the open nostril and release a single spray while ensuring that the subject inhales softly.
- Allow 30-60 seconds between sprays to avoid mucosal oversaturation.
Repeat as needed for additional doses. Wipe the nozzle clean using an alcohol swab after each use.
Researchers opting for an injectable formulation should consider that injectable N-Acetyl Selank Amidate is typically shipped as a vial of lyophilized powder.
Researchers will need to reconstitute the peptide powder with a suitable solvent prior to use and have all necessary laboratory materials on hand, including alcohol swabs, syringes and needles, and a solvent like bacteriostatic water.
Is N-Acetyl Selank Amidate Legal?
N-Acetyl Selank Amidate can be acquired legally for research purposes. It is not authorized for human use outside of Russia and Ukraine, where it is indicated for the treatment of generalized anxiety disorder.
To abide by applicable laws and regulations, researchers are encouraged to familiarize themselves with local laws governing the possession, distribution, and importation of research peptides like N-Acetyl Selank Amidate.
This includes regulations concerning research chemicals, controlled substances, and pharmaceuticals within the researcher’s jurisdiction.
N-Acetyl Selank Amidate Review
N-Acetyl Selank Amidate is a modified version of the potent anxiolytic and nootropic peptide Selank. It is posited to have similar effects and enhanced stability relative to the parent molecule.
Like Selank, N-Acetyl Selank Amidate may also have significant potential for reducing anxiety, improving tolerance to stress, and boosting cognitive function. Based on Selank research, N-Acetyl Selank Amidate is also expected to have a favorable safety profile.
Investigators interested in delving into the potential of N-Acetyl Selank Amidate are advised to visit our recommended online vendor of research peptides.
References
- Kolomin, T., Shadrina, M., Slominsky, P., Limborska, S., & Myasoedov, N. (2013). A new generation of drugs: synthetic peptides based on natural regulatory peptides. Neuroscience and Medicine, 4(04), 223-252.
- Vyunova, T. V., Andreeva, L., Shevchenko, K., & Myasoedov, N. (2018). Peptide-based Anxiolytics: The Molecular Aspects of Heptapeptide Selank Biological Activity. Protein and peptide letters, 25(10), 914–923. https://doi.org/10.2174/0929866525666180925144642
- Vasil'eva, E. V., Kondrakhin, E. A., Salimov, R. M., & Kovalev, G. I. (2016). Eksperimental'naia i klinicheskaia farmakologiia, 79(9), 3–11.
- Shevchenko, K. V., Nagaev, I. Y., Andreeva, L. A., Shevchenko, V. P., & Myasoedov, N. F. (2019). Prospects for Intranasal Delivery of Neuropeptides to the Brain. Pharmaceutical Chemistry Journal, 53, 89-100.
- Markov, D. D., Dolotov, O. V., & Grivennikov, I. A. (2023). The Melanocortin System: A Promising Target for the Development of New Antidepressant Drugs. International journal of molecular sciences, 24(7), 6664. https://doi.org/10.3390/ijms24076664
- Medvedev, V. E., Tereshchenko, O. N., Israelian, A. I.u, Chobanu, I. K., Kost, N. V., Sokolov, O. I.u, & Miasoedov, N. F. (2014). Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova, 114(7), 17–22.
- Semenova, T. P., Kozlovskiĭ, I. I., Zakharova, N. M., & Kozlovskaia, M. M. (2010). Eksperimental'naia i klinicheskaia farmakologiia, 73(8), 2–5.
- Uchakina, O. N., Uchakin, P. N., Miasoedov, N. F., Andreeva, L. A., Shcherbenko, V. E., Mezentseva, M. V., Gabaeva, M. V., Sokolov, O. I.u, Zozulia, A. A., & Ershov, F. I. (2008). Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova, 108(5), 71–75.
- Semenova, T. P., kozlovskiĭ, I. I., Zakharova, N. M., & Kozlovskaia, M. M. (2009). Eksperimental'naia i klinicheskaia farmakologiia, 72(4), 6–8.
- Kost, N. V., Sokolov, O. I.u, Gabaeva, M. V., Grivennikov, I. A., Andreeva, L. A., Miasoedov, N. F., & Zozulia, A. A. (2001). Ingibiruiushchee deĭstvie semaksa i selanka na énkefalindegradiruiushchie fermenty syvorotki krovi cheloveka [Semax and selank inhibit the enkephalin-degrading enzymes from human serum]]. Bioorganicheskaia khimiia, 27(3), 180–183. https://doi.org/10.1023/a:1011373002885
- Le Merrer, J., Becker, J. A., Befort, K., & Kieffer, B. L. (2009). Reward processing by the opioid system in the brain. Physiological reviews, 89(4), 1379–1412. https://doi.org/10.1152/physrev.00005.2009
- Zozulia, A. A., Neznamov, G. G., Siuniakov, T. S., Kost, N. V., Gabaeva, M. V., Sokolov, O. I.u, Serebriakova, E. V., Siranchieva, O. A., Andriushenko, A. V., Telesheva, E. S., Siuniakov, S. A., Smulevich, A. B., Miasoedov, N. F., & Seredenin, S. B. (2008). Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova, 108(4), 38–48.
- Inozemtseva, L. S., Karpenko, E. A., Dolotov, O. V., Levitskaya, N. G., Kamensky, A. A., Andreeva, L. A., & Grivennikov, I. A. (2008). Intranasal administration of the peptide Selank regulates BDNF expression in the rat hippocampus in vivo. Doklady biological sciences : proceedings of the Academy of Sciences of the USSR, Biological sciences sections, 421, 241–243. https://doi.org/10.1134/s0012496608040066
- Narkevich, V. B., Kudrin, V. S., Klodt, P. M., Pokrovskiĭ, A. A., Kozlovskaia, M. M., Maĭskiĭ, A. I., & Raevskiĭ, K. S. (2008). Eksperimental'naia i klinicheskaia farmakologiia, 71(5), 8–12.
- Medvedev, V. E., Tereshchenko, O. N., Kost, N. V., Ter-Israelyan, A. Y., Gushanskaya, E. V., Chobanu, I. K., Sokolov, O. Y., & Myasoedov, N. F. (2015). Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova, 115(6), 33–40. https://doi.org/10.17116/jnevro20151156133-40
- Leonidovna, Y. A., Aleksandrovna, S. M., Aleksandrovna, T. A., Aleksandrovna, B. O., Fedorovich, M. N., & Aleksandrovna, A. L. (2021). The Influence of Selank on the Level of Cytokines Under the Conditions of "Social" Stress. Current reviews in clinical and experimental pharmacology, 16(2), 162–167. https://doi.org/10.2174/1574884715666200704152810
- Uchakina, O. N., Uchakin, P. N., Miasoedov, N. F., Andreeva, L. A., Shcherbenko, V. E., Mezentseva, M. V., Gabaeva, M. V., Sokolov, O. I.u, Zozulia, A. A., & Ershov, F. I. (2008). Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova, 108(5), 71–75.
- Panchaprateep, R., Korkij, W., & Asawanonda, P. (2011). Brain-derived nerve factor and neurotrophins in androgenetic alopecia. The British journal of dermatology, 165(5), 997–1002. https://doi.org/10.1111/j.1365-2133.2011.10514.x
- Doyno, C. R., & White, C. M. (2021). Sedative-Hypnotic Agents That Impact Gamma-Aminobutyric Acid Receptors: Focus on Flunitrazepam, Gamma-Hydroxybutyric Acid, Phenibut, and Selank. Journal of clinical pharmacology, 61 Suppl 2, S114–S128. https://doi.org/10.1002/jcph.1922
- Селанк, капли назальные 0,15 % 3 мл 1 шт [In Russian] (n.d.) Available from:https://www.eapteka.ru/goods/id213328/
- Novak, E., Stubbs, S. S., Sanborn, E. C., & Eustice, R. M. (1972). The tolerance and safety of intravenously administered benzyl alcohol in methylprednisolone sodium succinate formulations in normal human subjects. Toxicology and applied pharmacology, 23(1), 54–61. https://doi.org/10.1016/0041-008x(72)90203-7
