Compound Overview
Class of Compound:
Non-peptide ghrelin agonist and growth hormone secretagogue.
Mechanism of Action:
MK-677 is an orally-active, selective agonist of the ghrelin receptor, known to increase the secretion of growth hormone and insulin-like growth factor 1, without stimulating serum cortisol levels.
Notable Studies:
- Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue
- MK-677, an orally active growth hormone secretagogue, reverses diet-induced catabolism
- Prolonged oral treatment with MK-677, a novel growth hormone secretagogue, improves sleep quality in man
Also Known As:
MK-0677, oratrope, ibutamoren, L-163,191.
Research Applications:
- Growth hormone deficiency
- Body composition
- Sleep quality
- Anti-aging
Risks:
- Increased appetite
- Lack of human trials
- Lack of FDA approval
Chemical Structure
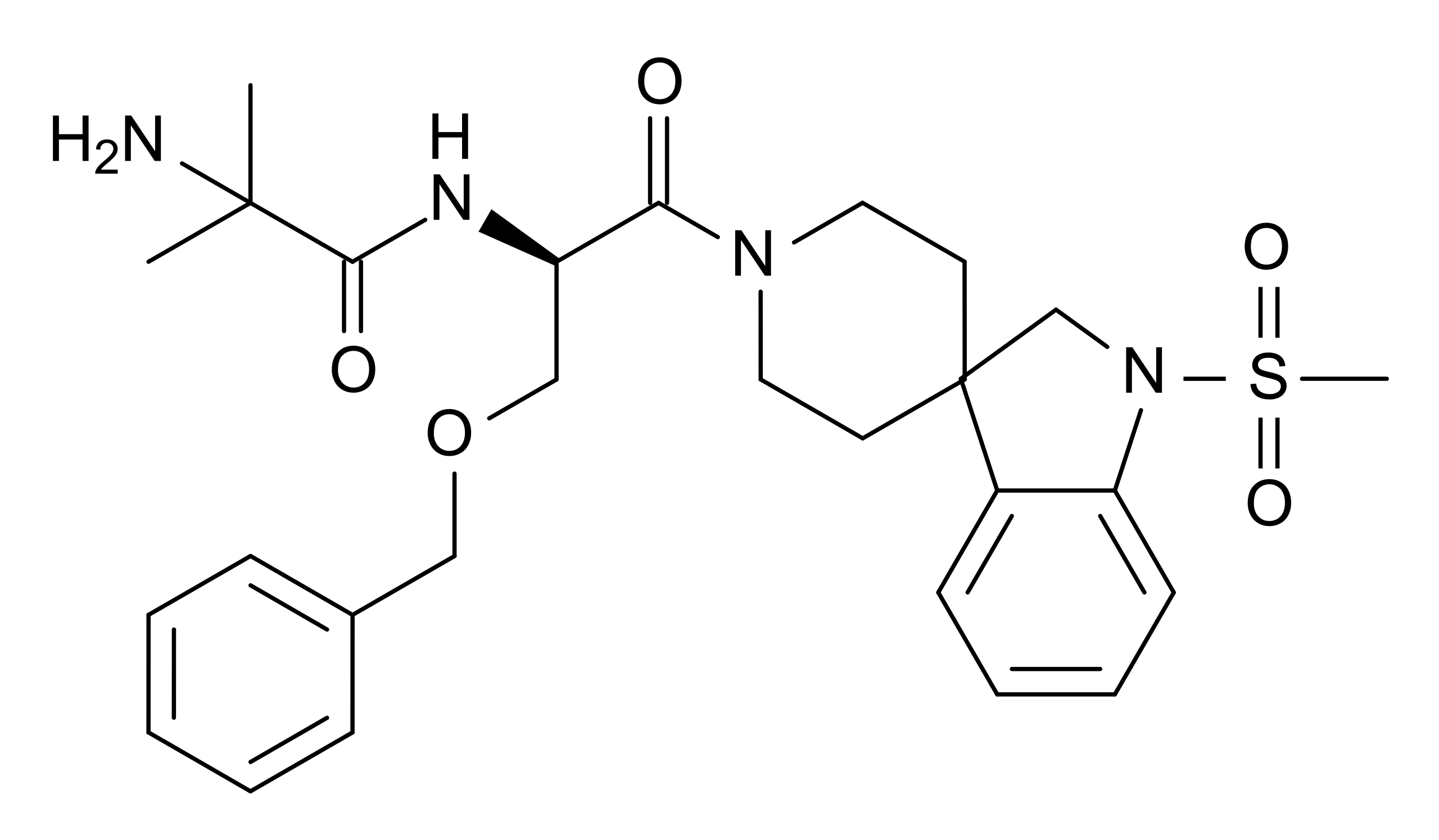
What is MK-677?
MK-677 refers to ibutamoren mesylate, an orally active, non-peptide growth hormone secretagogue. As a selective agonist of the ghrelin receptor, MK-677 stimulates the pituitary gland to produce and release growth hormone (GH) and insulin-like growth factor 1 (IGF-1) without affecting levels of cortisol to a significant extent [1].
MK-677 has been nominated as a treatment of growth hormone deficiency (GHD, including adult-onset GHD) and catabolic conditions, and has been extensively studied in a variety of contexts, including for its roles in promoting bone formation and resorption, aiding in fat loss, and improving sleep quality [2].
At the time of writing, there are no FDA-approved products containing ibutamoren mesylate, and MK-677 is classed as an Investigational New Drug in the United States, available to researchers as a reference material [2].
What Does MK-677 Do?
MK-677 is mechanistically indistinguishable from growth hormone-releasing peptide-6 (GHRP-6) and works by mimicking ghrelin, thus binding to the growth hormone secretion receptors (GHS-R) and stimulating the release of GH [3].
Preclinical animal studies have shown that MK-677 does not affect normal negative feedback mechanisms in the GH pathway such as somatostatin. MK-677 has been shown to trigger GH secretion without having a significant effect on plasma levels of aldosterone, luteinizing hormone, thyroxine, and prolactin, and only a modest effect on cortisol [1].
MK-677 is orally available, has an elimination half-life of 4-6 hours [1], and can be administered once daily [3], making it a strong candidate to treat GH deficiency as an alternative to exogenous GH therapy [4, 5].
MK-677 Benefits | Clinical Trials
Researchers at the University of Maryland have identified 16 experimental MK-677 studies and one observational study published between 1996 and 2018. These 17 studies involved a total of 1,664 test subjects, with the number of participants in each study varying between 8 and 564 [2]. Here is a summary of the main benefits of MK-677 observed:
Improves body composition: Several short-term clinical studies have found that MK-677 can positively impact body composition in test subjects. Notably:
A two-month study by Svensson et al. (1998) found that MK-677 helped increase GH levels and preserve levels of fat-free mass (FFM) when administered to twenty-four obese males while dieting. During the study, test subjects were assigned to receive either MK-677 25 mg or a placebo daily for 8 weeks.
Those who received MK-677 experienced a 40 percent increase in serum insulin-like growth factor I (IGF-I) and a significant increase in serum IGF-binding protein-3, especially during the second and eighth weeks. According to the researchers, MK-677 treatment resulted in sustained increases in lean body mass and a temporary increase in basal metabolic rate, levels suggesting that further study was needed to see if a higher dose of MK-677 or a longer treatment period would affect levels of body fat [7].
Enhances quality and duration of sleep: In 1997, Copinschi et al. found that MK-677 improved the quality and duration of sleep when administered to eight young test subjects. The study involved eight young test subjects aged 18-30 years participating in three treatment periods lasting seven days each.
The test subjects were randomly assigned to receive a bedtime dose of either MK-677 (5 and 25 mg) or placebo. Those who received MK-677 experienced a “50% increase in the duration of stage IV sleep and a more than 20% increase in REM sleep” compared to placebo. Researchers concluded that MK-677 may “simultaneously improve sleep quality and correct the relative hyposomatotropism of senescence” [8].
Aids bone formation and resorption: Other studies have investigated the effects of MK-677 on bone formation and resorption:
A 1999 paper published by Murphy et al. as “MK-677 Study Group” reported the findings from three separate studies into the effects of MK-677 on bone turnover in healthy and functionally impaired elderly adults.
The first study involved 32 healthy seniors who were assigned to receive either MK-677 at 10 mg or 25 mg, or placebo.
The second study involved 50 healthy adults aged 65-85 who received either MK-677 at 25 or 50 mg/day or placebo, while the third study involved 105 elderly patients with functional impairment aged 65-94 who received MK-677 (5, 10, or 25 mg) or placebo. According to the authors, “once-daily dosing of MK-677 stimulates bone turnover in elderly adults” [9].
A randomized, double-blind, parallel, and placebo-controlled trial by Svensson et al. (1999) assigned 24 obese patients to receive either MK-677 25 mg or placebo. Researchers found that short-term MK-677 treatment led to increases in markers of bone resorption and formation but suggested that further long-term studies were needed to gauge MK-677’s effect on bone mass [10].
Experimental treatment of Alzheimer’s Disease: A 2008 study by Sevigny et al. examined whether MK-677 could slow the rate of progression of symptoms in patients with Alzheimer’s Disease (AD). The researchers were operating on the belief that MK-677's ability to raise IGF-1 levels would clear amyloid beta plaques from the nervous systems of AD subjects, thus slowing the disease’s progression.
The study involved a total of 563 patients with mild to moderate AD who were randomly assigned to receive MK-677 25 mg or placebo daily for 12 months. 416 patients completed the study, and those in the MK-677 group experienced a 60 percent increase in serum IGF-1 levels at six months and a 72.9 percent increase after one year. Unfortunately, scores from the diagnostic tests used to assess the volunteers found that MK-677 was “ineffective at slowing the rate of progression of AD” [11].
Buy MK-677 from our #1 recommended vendor...

MK-677 Side Effects
The results of a study into the effects of MK-677 on healthy, older adults showed that it causes the following side effects [13]:
- Increased fasting blood glucose levels (0.3 mmol/L, 5 mg/dL on average)
- Decreased insulin sensitivity
- Increased appetite (typically subsides within a few months)
- Transient, mild lower extremity edema and muscle pain
- Decreased low density lipoprotein cholesterol
- Increased cortisol levels
This is consistent with the results of other MK-677 human clinical trials to date, which have not linked the compound to any severe side effects.
Is MK-677 Safe?
Little is known about the long-term safety of MK-677 as the drug has yet to pass a phase 2 trial and further research is needed to confirm its safety.
The most recent phase 2 trial involving MK-677 aimed to investigate the safety and efficacy of MK-677 for treating somatropin deficiency in children. However, this trial was suspended in July of 2021, before reaching a conclusion [4].
Notably, one 24-week study involving 123 elderly hip fracture patients was discontinued due to MK-677’s potential to increase the rate of congestive heart failure (CHF) in a few of the patients, with researchers concluding that the compound had an unfavorable safety profile in that specific population [14].
These findings indicate that a long-term safety study with independent validation is required before drawing any conclusions about the safety of MK-677.
MK-677 Dosage Calculator
Because MK-677 is an experimental compound with no officially recommended dosage, researchers may consult the above-cited literature to see how it has been administered in past studies.
Researchers typically start with an MK-677 dose of 12.5-25 mg/day across a variety of objectives.
Since it takes some time to achieve GH increase in the subject, the compound should be administered for an extended duration. Commonly, the material is given in cycles of 16-20 weeks, followed by a break of about one month.
Where to Buy MK-677 Online? | 2024 Edition
Qualified researchers may purchase MK-677 as a reference material from a number of online vendors. In our team’s experience, very few suppliers actually supply research-grade MK-677 or reliably deliver it worldwide.
Fortunately, we have found two vendors that do supply high-quality MK-677.
Chemyo
Chemyo is one of our top recommended vendors of MK-677, and here’s why:
- Independently-Tested Compounds: Each batch of MK-677 sold by Chemyo is tested in a third-party lab and the reports are published online for full transparency. This lets researchers verify the purity of what they’re buying before they place an order.
- Reasonable Prices: Chemyo sells research-grade MK-677 50mL (25mg/mL) for just $80, and researchers can save 10% when subscribing to the vendor’s newsletter.
- Convenient Payments: Researchers will be pleased to know that Chemyo accepts major credit cards, eChecks, and BTC.
Buy MK-677 from our top-rated vendor...

Limitless Life
Limitless Life is another extremely competent research chemicals vendor, and one that we trust based on their quality analysis, express shipping, and wonderful customer care team.
Below is why they are so trusted:
- Quality MK-677: Limitless Life’s third-party lab tests include HPLC-MS analysis to make certain that each lot of MK-677 is suitably pure and potent.
- Very Fast Shipping: Limitless Life guarantees delivery within 24-48 hours when using their express shipping method, with other, less efficient, options also available.
- Newsletter Offer: By signing up to the Limitless Life newsletter, researchers can receive a plentiful 10% off of their first order.
Buy research peptides from GOLDEN Peptides, a top-rated vendor...

FAQ
MK-677 | Reviews
MK-677 is an actively researched growth hormone secretagogue that has been shown to increase GH and IGF-I levels. There is evidence that it stimulates the production of fat-free mass, improves sleep and bone turnover, and reduces diet-induced nitrogen wasting—among exhibiting other benefits.
Given its generally favorable safety profile to date and ease of administration, MK-677 is a strong candidate for research in the areas of fat reduction, bone health, sleep, neurologic function, and longevity.
Researchers interested in studying MK-677 are advised to visit our preferred vendor.
References
- Patchett AA, Nargund RP, Tata JR, Chen MH, Barakat KJ, Johnston DB, Cheng K, Chan WW, Butler B, Hickey G, et al. Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):7001-5. doi: 10.1073/pnas.92.15.7001. PMID: 7624358; PMCID: PMC41459.
- Yoon, S., Gianturco, S., Pavlech, L., Storm, K., Yuen, M., & Mattingly, A. (2022). Ibutamoren mesylate: Summary Report. Retrieved 7 May 2022, from https://archive.hshsl.umaryland.edu/bitstream/handle/10713/14872/Ibutamoren%20mesylate_Final_2021_02.pdf?sequence=1&isAllowed=y
- Bowers CY. Growth hormone-releasing peptide (GHRP). Cell Mol Life Sci. 1998 Dec;54(12):1316-29. doi: 10.1007/s000180050257. PMID: 9893708.
- Ibutamoren - Lumos Pharma/Merck - AdisInsight. (2022). Retrieved 6 January 2022, from https://adisinsight.springer.com/drugs/800007434
- Sigalos JT, Pastuszak AW. The Safety and Efficacy of Growth Hormone Secretagogues. Sex Med Rev. 2018 Jan;6(1):45-53. doi: 10.1016/j.sxmr.2017.02.004. Epub 2017 Apr 8. PMID: 28400207; PMCID: PMC5632578.
- Murphy MG, Plunkett LM, Gertz BJ, He W, Wittreich J, Polvino WM, Clemmons DR. MK-677, an orally active growth hormone secretagogue, reverses diet-induced catabolism. J Clin Endocrinol Metab. 1998 Feb;83(2):320-5. doi: 10.1210/jcem.83.2.4551. PMID: 9467534.
- Svensson J, Lönn L, Jansson JO, Murphy G, Wyss D, Krupa D, Cerchio K, Polvino W, Gertz B, Boseaus I, Sjöström L, Bengtsson BA. Two-month treatment of obese subjects with the oral growth hormone (GH) secretagogue MK-677 increases GH secretion, fat-free mass, and energy expenditure. J Clin Endocrinol Metab. 1998 Feb;83(2):362-9. doi: 10.1210/jcem.83.2.4539. PMID: 9467542.
- Copinschi G, Leproult R, Van Onderbergen A, Caufriez A, Cole KY, Schilling LM, Mendel CM, De Lepeleire I, Bolognese JA, Van Cauter E. Prolonged oral treatment with MK-677, a novel growth hormone secretagogue, improves sleep quality in man. Neuroendocrinology. 1997 Oct;66(4):278-86. doi: 10.1159/000127249. PMID: 9349662.
- Murphy MG, Bach MA, Plotkin D, Bolognese J, Ng J, Krupa D, Cerchio K, Gertz BJ. Oral administration of the growth hormone secretagogue MK-677 increases markers of bone turnover in healthy and functionally impaired elderly adults. The MK-677 Study Group. J Bone Miner Res. 1999 Jul;14(7):1182-8. doi: 10.1359/jbmr.1999.14.7.1182. PMID: 10404019.
- Svensson J, Ohlsson C, Jansson JO, Murphy G, Wyss D, Krupa D, Cerchio K, Polvino W, Gertz B, Baylink D, Mohan S, Bengtsson BA. Treatment with the oral growth hormone secretagogue MK-677 increases markers of bone formation and bone resorption in obese young males. J Bone Miner Res. 1998 Jul;13(7):1158-66. doi: 10.1359/jbmr.1998.13.7.1158. PMID: 9661080.
- Sevigny JJ, Ryan JM, van Dyck CH, Peng Y, Lines CR, Nessly ML; MK-677 Protocol 30 Study Group. Growth hormone secretagogue MK-677: no clinical effect on AD progression in a randomized trial. Neurology. 2008 Nov 18;71(21):1702-8. doi: 10.1212/01.wnl.0000335163.88054.e7. PMID: 19015485.
- Jeong YO, Shin SJ, Park JY, Ku BK, Song JS, Kim JJ, Jeon SG, Lee SM, Moon M. MK-0677, a Ghrelin Agonist, Alleviates Amyloid Beta-Related Pathology in 5XFAD Mice, an Animal Model of Alzheimer's Disease. Int J Mol Sci. 2018 Jun 18;19(6):1800. doi: 10.3390/ijms19061800. PMID: 29912176; PMCID: PMC6032329.
- Nass, Ralf et al. “Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial.” Annals of internal medicine vol. 149,9 (2008): 601-11. doi:10.7326/0003-4819-149-9-200811040-00003
- Adunsky A, Chandler J, Heyden N, Lutkiewicz J, Scott BB, Berd Y, Liu N, Papanicolaou DA. MK-0677 (ibutamoren mesylate) for the treatment of patients recovering from hip fracture: a multicenter, randomized, placebo-controlled phase IIb study. Arch Gerontol Geriatr. 2011 Sep-Oct;53(2):183-9. doi: 10.1016/j.archger.2010.10.004. Epub 2010 Nov 9. PMID: 21067829.
